An annulative transfer hydrogenation strategy enables straightforward access to tetrahydro fused-pyrazine derivatives†
Abstract
A ruthenium-catalysed annulative transfer hydrogenation strategy, enabling straightforward access to tetrahydro fused-pyrazine derivatives from N-heteroaryl diamines and vicinal diols, has been demonstrated for the first time. Such a synthesis proceeds with unprecedented synthetic effectiveness including high step- and atom efficiency, generation of water as the sole by-product, short reaction time and no need for external high pressure H2 gas, offering an important basis for the transformation of vicinal diols, a class of bio-mass derived resources, into functionalized products.
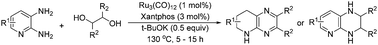

 Please wait while we load your content...
Please wait while we load your content...