Intermolecular iodofunctionalization of allenamides with indoles, pyrroles, and furans: synthesis of iodine-substituted Z-enamides†
Abstract
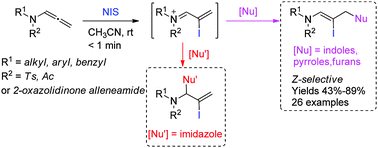
A new method was developed to synthesize iodine-substituted Z-enamides through N-iodosuccinimide-mediated intermolecular iodofunctionalization of allenamides with indoles, pyrroles, and furans. These reactions proceed rapidly and tolerate a broad scope of substrates. The conjugated sulfimide ion species probably acts as the key intermediate.

 Please wait while we load your content...
Please wait while we load your content...