The amide C–N bond of isatins as the directing group and the internal oxidant in Ru-catalyzed C–H activation and annulation reactions: access to 8-amido isocoumarins†
Abstract
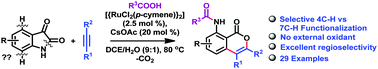
The N–O, N–N and O–O bonds are the frequently used internally oxidative directing groups used in various redox-neutral coupling reactions. The sole use of the C–N bond as the oxidizing directing group was reported recently by Li X. and co-workers for the Rh(III)-catalyzed C–H activation of phenacyl ammonium salts. Herein, we report the use of the amide C–N bond of isatins as the oxidizing directing group for the Ru(II)-catalyzed redox-neutral C–H activation and annulation reactions with alkynes which afford 8-amido isocoumarins. The reaction also features excellent regioselectivity with alkyl aryl substituted alkynes.

 Please wait while we load your content...
Please wait while we load your content...