Consecutive visible-light photoredox decarboxylative couplings of adipic acid active esters with alkynyl sulfones leading to cyclic compounds†
Abstract
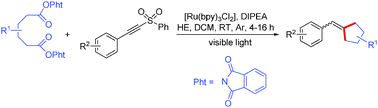
Novel and efficient consecutive photoredox decarboxylative couplings of adipic acid active esters (bis(1,3-dioxoisoindolin-2-yl)-substituted hexanedioates) with substituted 1-(2-arylethynylsulfonyl)benzenes have been developed under visible-light photocatalysis. The successive photoredox decarboxylative C–C bond formation at room temperature afforded the corresponding cyclic compounds in good yields with tolerance of some functional groups.

 Please wait while we load your content...
Please wait while we load your content...