Organocatalytic silyl transfer from silylborane to nitroalkenes for the synthesis of β-silyl nitroalkanes and β-silyl amines†
Abstract
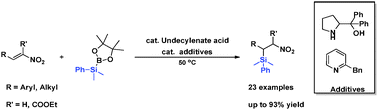
The silylation of a wide array of nitroalkenes is achieved by organocatalysts in yields up to 93%. The reaction is carried out in a toluene/water biphasic solvent under operationally simple conditions. Reduction of the nitro group provides efficient access to functionalized β-silyl amines.

 Please wait while we load your content...
Please wait while we load your content...