A rapidly photo-activatable light-up fluorescent nucleoside and its application in DNA base variation sensing†
Abstract
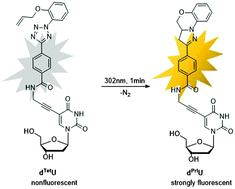
A new DNA building block (dTetU) bearing a tetrazole and allyloxy group at N-phenyl ring linked through an aminopropynyl linker to the 5-position of 2′-deoxyuridine was synthesized. The modified DNA can be lit up via a photoinduced intramolecular tetrazole–alkene cycloaddition reaction, but quenched when the fully-matched double strand is formed. This conspicuous difference in fluorescence could open a door for DNA single nucleotide polymorphism (SNP) typing.

 Please wait while we load your content...
Please wait while we load your content...