Quinonediimines as redox-active organocatalysts for oxidative coupling of aryl- and alkenylmagnesium compounds under molecular oxygen†
Abstract
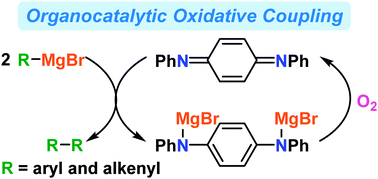
It is revealed that N,N′-diphenyl-p-benzoquinonediimine works as a redox-active organocatalyst for the oxidative homo-coupling of aryl- and alkenylmagnesium compounds under molecular oxygen. The catalytic cycle was formally monitored by 1H NMR experiments.

 Please wait while we load your content...
Please wait while we load your content...