PdII/CuBr2 catalysed keto α-Csp3–H benzoxylation of N,N-dialkylamides directed by o-hydroxy groups†
Abstract
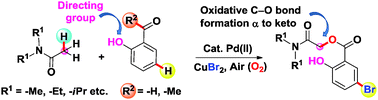
A hydroxy group directed keto α-Csp3–H benzoxylation of amides, including N,N-dialkylamides and cyclic amides, has been accomplished involving ortho-hydroxy substrates possessing either an aldehydic or a keto methyl (–COCH3) group with a Pd(II)/CuBr2 catalytic combination. The carboxy group obtained via the in situ oxidation of –CHO or –COCH3 groups of ortho-hydroxy substrates then undergoes a cross-dehydrogenative coupling (CDC) with amides to furnish an α-benzoxylation product with concurrent aromatic ring bromination.

 Please wait while we load your content...
Please wait while we load your content...