An iron-based thin film as a highly efficient catalyst for electrochemical water oxidation in a carbonate electrolyte†
Abstract
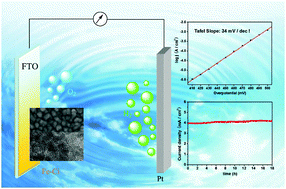
A highly active iron-based water-oxidation catalyst was electrodeposited from a CO2 saturated bicarbonate solution containing Fe2+. This catalyst (Fe-Ci) produces a current density of 10 mA cm−2 at an overpotential of 560 mV and the activity remains constant over 18 h in the environmentally benign HCO3−/CO32− buffer (pH 9.75). A Tafel slope of 34 mV dec−1 was obtained for Fe-Ci, which is lower than other reported values for iron-based catalysts.

 Please wait while we load your content...
Please wait while we load your content...