Electronic interactions of i, i + 1 dithioamides: increased fluorescence quenching and evidence for n-to-π* interactions†
Abstract
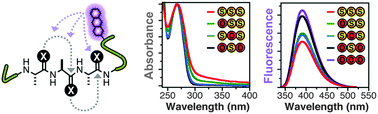
Thioamide residues can be effective, minimally-perturbing fluorescence quenching probes for studying protein folding and proteolysis. In order to increase the level of quenching, we have here explored the use of adjacent dithioamides. We have found that they are more effective fluorescence quenchers, as expected, but we have also observed unexpected changes in the thioamide absorption spectra that may arise from n-to-π* interactions of the thiocarbonyls. We have made use of the increased quenching to improve the fluorescence turn-on of thioamide protease sensors.
- This article is part of the themed collection: Foldamers

 Please wait while we load your content...
Please wait while we load your content...