Rupture force of cell adhesion ligand tethers modulates biological activities of a cell-laden hydrogel†
Abstract
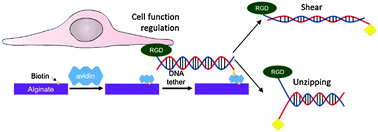
Recent efforts to design a synthetic extracellular matrix for cell culture, engineering, and therapies greatly contributed to addressing biological roles of types and spatial organization of cell adhesion ligands. It is often suggested that ligand–matrix bond strength is another path to regulate cell adhesion and activities; however tools are lacking. To this end, this study demonstrates that a hydrogel coupled with integrin-binding deoxyribonucleic acid (DNA) tethers with pre-defined rupture forces can modulate cell adhesion, differentiation, and secretion activities due to the changes in the number and, likely, force of cells adhered to a gel. The rupture force of DNA tethers was tuned by altering the spatial arrangement of matrix-binding biotin groups. The DNA tethers were immobilized on a hydrogel of alginate grafted with biotin using avidin. Mesenchymal stem cells showed enhanced adhesion, neural differentiation, and paracrine secretion when cultured on the gel coupled with DNA tethers with higher rupture forces. Such innovative cell–matrix interface engineering would be broadly useful for a series of materials used for fundamental and applied studies on biological cells.


 Please wait while we load your content...
Please wait while we load your content...