Dual nucleophilic substitution at a W(ii) η2-coordinated diiodo acetylene leading to an amidinium carbyne complex†
Abstract
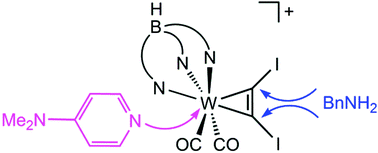
The synthesis and reactivity of a W(II) C2I2 complex towards various nucleophiles are described. Soft, aprotic nucleophiles like 4-dimethylaminopyridine (DMAP) lead to substitution of one CO at tungsten, whereas reaction with an excess of benzylamine results in a dual nucleophilic substitution at the alkyne moiety involving the rearrangement to a novel cationic amidinium carbyne complex.

 Please wait while we load your content...
Please wait while we load your content...