Construction of 1-pyrroline skeletons by Lewis acid-mediated conjugate addition of vinyl azides†
Abstract
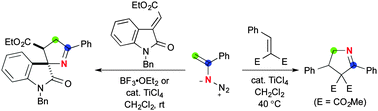
Lewis acid-mediated conjugate addition of vinyl azides to electron-deficient alkenes led to the efficient construction of 1-pyrroline skeletons. The reactions of vinyl azides with 3-alkylidene-2-oxoindolines afford 3′,4′-dihydrospiro[indoline-3,2′-pyrrol]-2-ones in a diastereoselective fashion, whereas those with dimethyl 2-alkylidenemalonates provide 4,5-dihydro-3H-pyrroles.

 Please wait while we load your content...
Please wait while we load your content...