Selectivity switching resulting in the formation of benzene by surface carbonates on ceria in catalytic gas-phase oxidation of benzyl alcohol†
Abstract
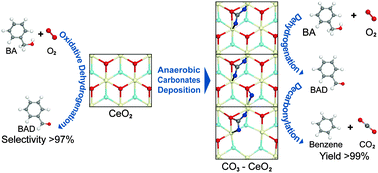
The irreversible formation of carbonate deposits on CeO2 leads to complete switching of catalytic selectivity resulting in the formation of benzene in gas-phase oxidation of benzyl alcohol. By integrating experimental spectra and theoretical calculations, we expect that such a shift is derived from further decarbonylation of benzaldehyde via easy trapping of CO fragments by surface carbonate species.

 Please wait while we load your content...
Please wait while we load your content...