The bioinspired design of a reagent allows the functionalization of Cα–H of α,β-unsaturated carbonyl compounds via the Baylis–Hillman chemistry under ambient conditions†
Abstract
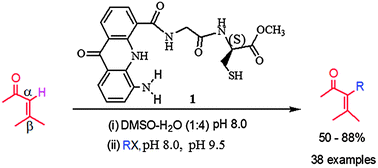
A rationally designed reagent capable of affecting alkylation at Cα of α,β-unsaturated carbonyl compounds is reported. The reaction proceeded at room temperature without any additives. The pH and H-bond formation during the reaction play a key role in the working of the reagent.

 Please wait while we load your content...
Please wait while we load your content...