Chiral Brønsted acid-catalysed enantioselective synthesis of isoindolinone-derived N(acyl),S-acetals†
Abstract
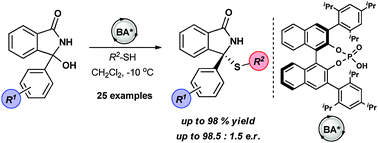
The first organocatalytic asymmetric addition of thiols to N-acyl ketimines, which are generated in situ from 3-hydroxy isoindolinones, is described. The reaction proceeds smoothly with a broad range of ketimines and thiols using a chiral Brønsted acid catalyst to afford N(acyl),S-acetals comprising a tetrasubstituted stereocenter in high yields and enantioselectivities (up to 98.5 : 1.5 e.r.). The usefulness of the developed protocol is demonstrated in the synthesis of a known HIV-1 reverse transcriptase inhibitor.

 Please wait while we load your content...
Please wait while we load your content...