Ammonium catalyzed cyclitive additions: evidence for a cation–π interaction with alkynes†
Abstract
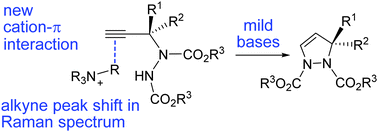
The addition of carbamate nitrogen to a non-conjugated carbon–carbon triple bond is catalyzed by an ammonium salt leading to a cyclic product. Studies in homogeneous systems suggest that the ammonium agent facilitates nitrogen–carbon bond formation through a cation–π interaction with the alkyne unit that, for the first time, is directly observed by Raman spectroscopy.

 Please wait while we load your content...
Please wait while we load your content...