The reduction of graphene oxide with hydrazine: elucidating its reductive capability based on a reaction-model approach†
Abstract
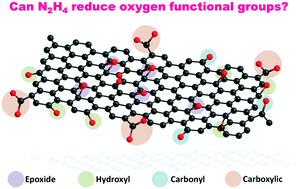
We have performed an experimental investigation on the effects of hydrazine treatment on graphene oxide via a reaction-model approach. Hydrazine was reacted with small conjugated aromatic compounds containing various oxygen functional groups to mimic the structure of graphene oxide. The hydroxyl and carboxylic groups were not readily removed while carbonyl groups reacted with hydrazine to form the corresponding hydrazone complexes. In the presence of adjacent hydroxyl groups, carboxyl groups underwent thermal decarboxylation.

 Please wait while we load your content...
Please wait while we load your content...