Conversion of a non-heme iron-dependent sulfoxide synthase into a thiol dioxygenase by a single point mutation†
Abstract
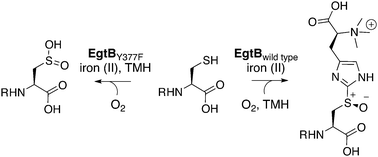
EgtB from Mycobacterium thermoresistibile catalyzes O2-dependent sulfur–carbon bond formation between the side chains of Nα-trimethyl histidine and γ-glutamyl cysteine as a central step in ergothioneine biosynthesis. A single point mutation converts this enzyme into a γ-glutamyl cysteine dioxygenase with an efficiency that rivals naturally evolved thiol dioxygenases.

 Please wait while we load your content...
Please wait while we load your content...