Switchable asymmetric bio-epoxidation of α,β-unsaturated ketones†
Abstract
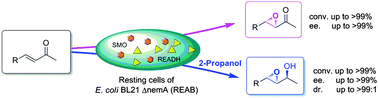
Efficient asymmetric bio-epoxidation of electron-deficient α,β-unsaturated ketones was realized via a tandem reduction-epoxidation-dehydrogenation cascade, which proceeds in a switchable manner to afford either chiral epoxy ketones or allylic epoxy alcohols with up to >99% yield and >99%ee.

 Please wait while we load your content...
Please wait while we load your content...