Electrophilic trifluoromethylation of carbonyl compounds and their nitrogen derivatives under copper catalysis†
Abstract
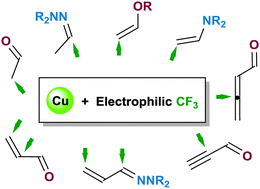
Recent advances in electrophilic trifluoromethylation reactions of carbonyl compounds and their usual surrogates are highlighted with particular focus on copper-catalysed (or mediated) C–CF3 bond forming reactions. Ketones and aldehydes (notably via their enol ether and enamine derivatives) enable electrophilic trifluoromethylation at the α-carbon of the carbonyl compounds, whereas aldehyde N,N-disubstituted hydrazones undergo electrophilic attack of the cationic or radical CF3 species at the azomethine carbon, thus providing an umpolung alternative to nucleophilic trifluoromethylation of carbonyl compounds. A reversal in reactivity is also observed for conjugated systems. While α,β-unsaturated ketones regioselectively incorporate the CF3 moiety at the α-position of the enones, trifluoromethylation occurs preferentially at the olefinic β-carbon of the corresponding hydrazones.

 Please wait while we load your content...
Please wait while we load your content...