Palladium-catalyzed three-component reaction of N-tosyl hydrazones, isonitriles and amines leading to amidines†
Abstract
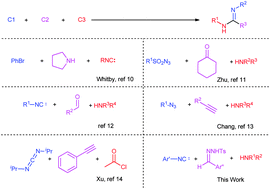
A palladium-catalyzed three-component reaction between N-tosyl hydrazones, aryl isonitriles and amines was developed, leading to amidines in moderate to good yields. This procedure features the rapid construction of amidine frameworks with high diversity and complexity. Ketenimines serve as intermediates, which encounter nucleophilic attack by amines to produce amidines.

 Please wait while we load your content...
Please wait while we load your content...