H2-driven biotransformation of n-octane to 1-octanol by a recombinant Pseudomonas putida strain co-synthesizing an O2-tolerant hydrogenase and a P450 monooxygenase†
Abstract
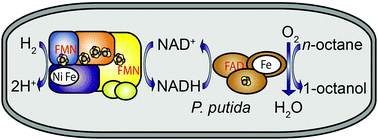
An in vivo biotransformation system is presented that affords the hydroxylation of n-octane to 1-octanol on the basis of NADH-dependent CYP153A monooxygenase and NAD+-reducing hydrogenase heterologously synthesized in a bacterial host. The hydrogenase sustains H2-driven NADH cofactor regeneration even in the presence of O2, the co-substrate of monooxygenase.

 Please wait while we load your content...
Please wait while we load your content...