Palladium(ii)-catalysed regioselective synthesis of 3,4-disubstituted quinolines and 2,3,5-trisubstituted pyrroles from alkenes via anti-Markovnikov selectivity†
Abstract
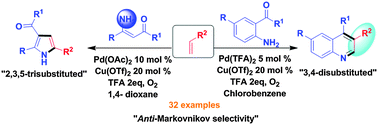
A novel strategy has been identified for the regioselective synthesis of 3,4-disubstituted quinolines and 2,3,5-trisubstituted pyrroles from simple alkenes via anti-Markovnikov selectivity under palladium catalysis. The salient features are synthesis of two different heterocycles, readily available starting materials, broad substrate scope, moderate to good yields and use of molecular oxygen as a terminal oxidant.

 Please wait while we load your content...
Please wait while we load your content...