Multicomponent reassembly of terpyridine-based materials: quantitative metallomacrocyclic rearrangement†
Abstract
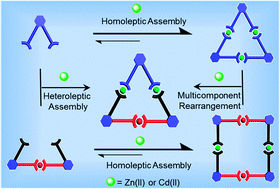
Mixing of metallocyclic trimers and tetramers in an exact 1 : 1.5 stoichiometry provided new supramolecular triangles in quantitative yields. Characterization of the new hetero-nuclear metallomacrocycles was achieved by 1H, 2D-COSY, 2D-NOESY, and 13C NMR spectroscopy, along with ESI and TWIM mass spectrometry. Gradient tandem MS (gMS2) provided insight into the stabilities of the binuclear structures.

 Please wait while we load your content...
Please wait while we load your content...