Enantio- and diastereoselective synthesis of γ-amino alcohols†
Abstract
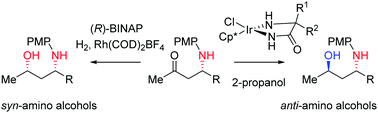
The γ-amino alcohol structural motif is often encountered in drugs and natural products. We developed two complementary catalytic diastereoselective methods for the synthesis of N-PMP-protected γ-amino alcohols from the corresponding ketones. The anti-products were obtained through Ir-catalyzed asymmetric transfer hydrogenation, the syn-products via Rh-catalyzed asymmetric hydrogenation.

 Please wait while we load your content...
Please wait while we load your content...