The long underestimated carbonyl function of carbohydrates – an organocatalyzed shot into carbohydrate chemistry
Abstract
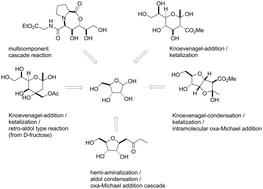
The aggressive and strong development of organocatalysis provides several protocols for the convenient utilization of the carbonyl function of unprotected carbohydrates in C–C-bond formation processes. These amine-catalyzed mechanisms enable multiple cascade-protocols for the synthesis of a wide range of carbohydrate-derived compound classes. Several, only slightly different protocols, have been developed for the application of 1,3-dicarbonyl compounds in the stereoselective chain-elongation of unprotected carbohydrates and the synthesis of highly functionalized C-glycosides of defined configuration. In addition, C-glycosides can also be accessed by amine-catalyzed reactions with methyl ketones. By a one-pot cascade reaction of isocyanides with unprotected aldoses and amino acids access to defined configured glycopeptide mimetics is achieved. Depending on the reaction conditions different origins to control the installation of configuration during the bond-formation process were observed.

 Please wait while we load your content...
Please wait while we load your content...