The synthesis of optically active N–C axially chiral tetrahydroquinoline and its response to an acid-accelerated molecular rotor†
Abstract
Optically active atropisomeric N-(2,5-di-tert-butylphenyl)-1,2,3,4-tetrahydroquinoline with an N–C chiral axis was prepared via a catalytic enantioselective reaction. The addition of methane sulfonic acid to this axially chiral quinoline dramatically lowered the barrier to rotation around the chiral axis.
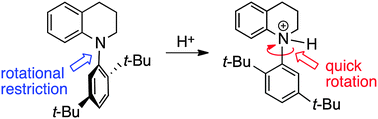

 Please wait while we load your content...
Please wait while we load your content...