Organocatalytic enantio- and diastereoselective synthesis of highly substituted δ-lactones via a Michael-cyclization cascade†
Abstract
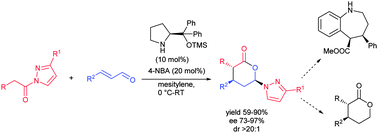
An organocatalyzed Michael-cyclization cascade approach of readily available α,β-unsaturated aldehydes and pyrazoleamides has been developed to get highly substituted δ-lactones in excellent enantioselectivities (up to 97%) and diastereoselectivities. The δ-lactones so obtained could easily be transformed into benzazepine derivatives with excellent enantio- and diastereoselectivities. Furthermore, the pyrazole moiety from the δ-lactones can be simply cleaved without disturbing the stereoselectivity.

 Please wait while we load your content...
Please wait while we load your content...