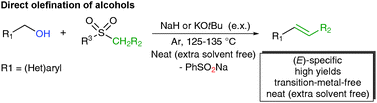
(E)-Specific direct Julia-olefination of aryl alcohols without extra reducing agents promoted by bases†
Abstract
An unprecedented base-promoted direct olefination of aryl alcohols with sulfones via a Julia-type reaction has been described. No extra reductants are needed for Julia reaction since alcohols work as double sources of aldehydes and the hydride. Generally high yields were given for both terminal and highly (E)-selective internal olefins.

 Please wait while we load your content...
Please wait while we load your content...