A general catalytic reaction sequence to access alkaloid-inspired indole polycycles†
Abstract
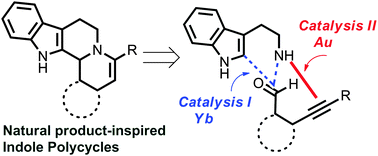
A catalytic two-step reaction sequence was developed to access a range of complex heterocyclic frameworks based on biorelevant indole/oxindole scaffolds. The reaction sequence includes catalytic Pictet–Spengler cyclization followed by Au(I) catalyzed intramolecular hydroamination of acetylenes. A related cascade polycyclization of a designed β-carboline embodying a 1,5-enyne group yields the analogues of the alkaloid harmicine.

 Please wait while we load your content...
Please wait while we load your content...