Methyltrioxorhenium-catalyzed highly selective dihydroxylation of 1,2-allenylic diphenyl phosphine oxides†
Abstract
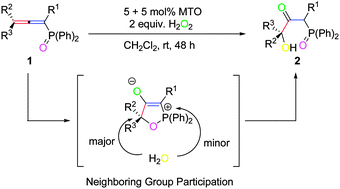
For the first time, methyltrioxorhenium (MTO) has been applied as a catalyst for the dihydroxylation of allenes in the presence of hydrogen peroxide as the oxidant. The regioselectivities turn out to be well controlled, affording β-carbonyl-γ-hydroxyl diphenyl phosphine oxides as the only product. The axial chirality of optically active allenes can also be nicely transferred to the chirality center of the products. Based on chirality transfer experiments and ESI-MS studies of 18O-labeled products, a possible mechanism, proceeding via regioselective epoxidation of the electron-rich carbon–carbon double bond, a subsequent intermolecular nucleophilic attack of a water molecule on the in situ formed epoxide via neighboring group participation (NGP), followed by a rearrangement has been proposed as the major reaction pathway.

 Please wait while we load your content...
Please wait while we load your content...