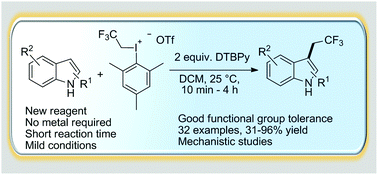
Abstract
A novel highly C3 selective metal free trifluoroethylation of indoles using 2,2,2-trifuoroethyl(mesityl)-iodonium triflate was developed. The methodology enables the introduction of a trifluoroethyl group in a fast and efficient reaction under mild conditions with high functional group tolerance. Beyond the synthetic developments, quantum chemical calculations provide a deeper understanding of the transformation.

 Please wait while we load your content...
Please wait while we load your content...