Enantioselective synthesis of dihydrocoumarin derivatives by chiral scandium(iii)-complex catalyzed inverse-electron-demand hetero-Diels–Alder reaction†
Abstract
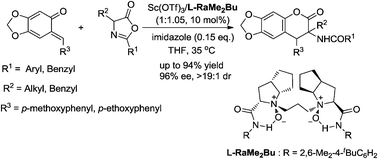
An asymmetric inverse-electron-demand hetero-Diels–Alder reaction between o-quinone methides and azlactones to generate potentially pharmacological active dihydrocoumarins has been achieved efficiently by using a chiral N,N′-dioxide–Sc(III) complex as the catalyst. The desired products were obtained in high yields with excellent enantioselectivities and diastereoselectivities (up to 94% yield, 96% ee and >19 : 1 dr) under mild reaction conditions. A concerted reaction pathway was confirmed by Operando IR and control experiments.

 Please wait while we load your content...
Please wait while we load your content...