Palladium-catalyzed oxidative carbamoylation of isoquinoline N-oxides with formylamides by means of dual C–H oxidative coupling†
Abstract
A new palladium-catalyzed oxidative carbamoylation reaction of isoquinoline N-oxides with formylamides for the synthesis of isoquinoline-1-carboxamides is established. The method represents the first example of the carbamoylation of isoquinoline N-oxides with formylamides to furnish arylamides using the dual C–H oxidation strategy.
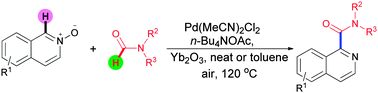

 Please wait while we load your content...
Please wait while we load your content...