Preparation of phenanthrenes from ortho-amino-biphenyls and alkynes via base-promoted homolytic aromatic substitution†
Abstract
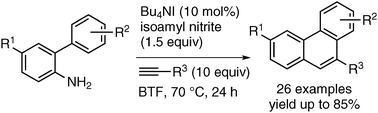
A transition-metal-free phenanthrene synthesis starting from readily accessible ortho-amino-biaryls is presented. The biarylamines are in situ transformed into the corresponding diazonium salts which upon single electron reduction give the corresponding aryl radicals. Addition to an alkyne and subsequent base promoted homolytic aromatic substitution (BHAS) provide phenanthrenes in moderate to good yields.

 Please wait while we load your content...
Please wait while we load your content...