An atom economical method for the direct synthesis of quinoline derivatives from substituted o-nitrotoluenes†
Abstract
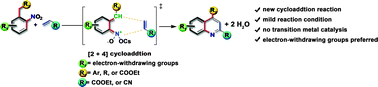
A highly efficient one-pot procedure for the preparation of substituted quinolines from substituted o-nitrotoluenes with electron-withdrawing groups and olefins (acrylic esters and acrylonitriles) using a cesium catalyst has been developed. A plausible [2+4] cycloaddition mechanism is proposed. This method uses nitroaromatic compounds as the starting materials to give quinoline derivatives in good to high yields under mild conditions with no transition metal catalysis. It provides an atom economical pathway for the synthesis of quinoline derivatives which could be used in industrial processes.

 Please wait while we load your content...
Please wait while we load your content...