A fast, efficient and simple method for the synthesis of cyclic alkenyl fluorides by a fluorinative carbocyclization reaction†
Abstract
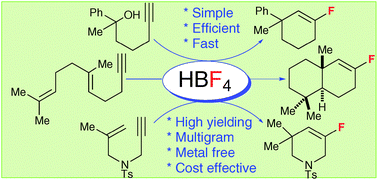
A simple transformation of alkynol or enyne derivatives into cyclic alkenyl fluorides by using tetrafluoroboric acid as the proton and fluoride source is reported. This study includes the first biomimetic cationic cyclization/nucleophilic fluorination reaction of polyenyne derivatives to give terpenoid derivatives.

 Please wait while we load your content...
Please wait while we load your content...