Direct enantiospecific substitution of primary α-aminoalkylferrocenes via Lewis acid-catalyzed C–N bond cleavage†
Abstract
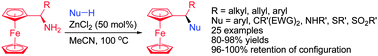
Highly enantioenriched primary α-aminoalkylferrocenes were found to undergo zinc chloride-catalyzed substitution with various carbon, nitrogen, and sulfur nucleophiles in an enantiospecific fashion through C–N bond cleavage. The reaction tolerates air and moisture and exhibits high atom-economy by releasing ammonia as the sole byproduct.

 Please wait while we load your content...
Please wait while we load your content...