Practical preparation of challenging amides from non-nucleophilic amines and esters under flow conditions†
Abstract
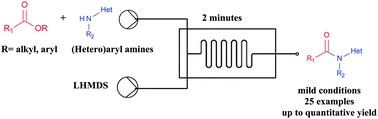
A fast and efficient protocol for the formation of amides from low nucleophilic amines and esters in flow is described. Products were obtained in good to excellent yields and with the advantage of simultaneous mixing of all reagents at once, avoiding steps for intermediate formation. The protocol is also suitable to be combined with ester synthesis, resulting in the preparation of amides in-line from haloarenes.
- This article is part of the themed collection: Recent Advances in Flow Synthesis and Continuous Processing

 Please wait while we load your content...
Please wait while we load your content...