An efficient generation method and remarkable reactivities of 3-triflyloxybenzyne†
Abstract
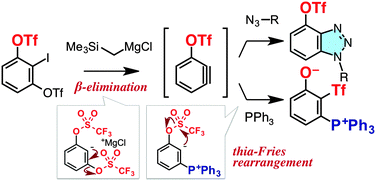
3-Triflyloxybenzyne was generated via an iodine–magnesium exchange reaction of 1,3-bis(triflyloxy)-2-iodobenzene using the trimethylsilylmethyl Grignard reagent. Various arynophiles and nucleophiles reacted regioselectively with 3-triflyoxybenzyne to afford cycloadducts and unique aryl triflones, which were provided via thia-Fries rearrangement.

 Please wait while we load your content...
Please wait while we load your content...