Retro-Claisen benzylation: direct use of benzyl alcohols in Pd-catalyzed couplings with nitriles†
Abstract
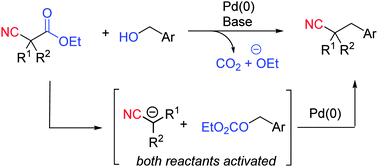
A new strategy has been developed for the benzylation of nitriles directly from benzyl alcohols. In this process benzyl alcohols undergo retro-Claisen activation with cyanoacetic esters to generate an active electrophile and a carbanionic nucleophile. In the presence of Pd(0) these intermediates undergo catalytic coupling to generate a new C–C bond, resulting in the formation of phenyl propionitriles.

 Please wait while we load your content...
Please wait while we load your content...