Total syntheses of (±)-musellarins A–C†
Abstract
The first, diastereoselective total syntheses of musellarins A–C were achieved concisely with 7.8–9.8% yields in 15–16 steps. The key synthetic features include (i) an Achmatowicz rearrangement, Kishi reduction, and Friedel–Crafts cyclization to construct the tricyclic framework and (ii) Heck coupling of aryldiazonium salts to introduce the aryl group into the dihydropyran in a 2,6-trans fashion in the final stage of synthesis.
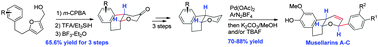

 Please wait while we load your content...
Please wait while we load your content...