New methods for the synthesis of naphthyl amines; application to the synthesis of dihydrosanguinarine, sanguinarine, oxysanguinarine and (±)-maclekarpines B and C†
Abstract
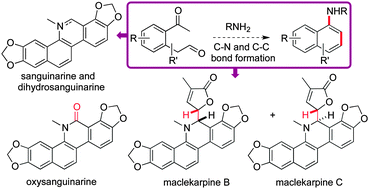
A new method for preparing naphthyl amines from 1,5 unsaturated dicarbonyl precursors is described; the utility of this new method was proven in the syntheses of several natural products, all containing the benzo[c]phenanthridine core and enabled by a radical promoted cyclisation of the naphthyl amine products formed in the key cyclisation.
- This article is part of the themed collection: In Celebration of Richard Taylor’s 65th Birthday

 Please wait while we load your content...
Please wait while we load your content...