An umpolung approach toward N-aryl nitrone construction: a phosphine-mediated addition of 1,2-dicarbonyls to nitroso electrophiles†
Abstract
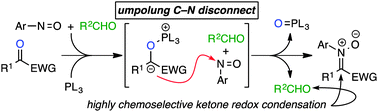
An umpolung approach toward nitrone construction utilizing a phosphine-mediated addition of 1,2-dicarbonyls to nitroso compounds is reported. The reaction exhibits a high degree of chemoselectivity and provides direct access to isoxazolidines, imines, and trisubstituted alkenes.

 Please wait while we load your content...
Please wait while we load your content...