Rhodium-catalyzed direct synthesis of unprotected NH-sulfoximines from sulfoxides†
Abstract
A novel rhodium-catalyzed imination of sulfoxides using O-(2,4-dinitrophenyl)hydroxylamine is developed under mild conditions with good functional group tolerance. This method provides an efficient access to free NH-sulfoximines, an important structural unit in a variety of biologically active compounds.
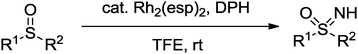

 Please wait while we load your content...
Please wait while we load your content...