Efficient synthesis of carbazolespirooxindole skeletons via asymmetric Diels–Alder reaction of 3-vinylindoles and methyleneindolinones†
Abstract
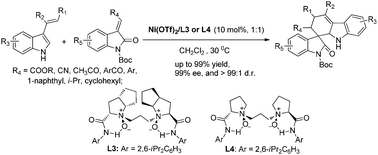
A highly efficient catalytic asymmetric Diels–Alder reaction between 3-vinylindoles and methyleneindolinones has been achieved using chiral N,N′-dioxide–Ni(II) complexes as the catalysts. A wide variety of substrates were readily tolerated, generating exclusively the corresponding exo-carbazolespirooxindole derivatives in excellent yields with high enantiomeric excesses (up to 98% yield, >99 : 1 d.r., and 98% ee) under mild reaction conditions.

 Please wait while we load your content...
Please wait while we load your content...