A homolytic oxy-functionalization mechanism: intermolecular hydrocarbyl migration from M–R to vanadate oxo†
Abstract
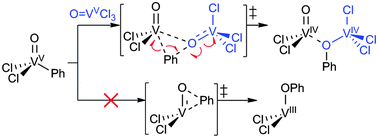
A new mechanism for generating C–O bonds from metal-hydrocarbyls involving homolytic, intermolecular migration of the hydrocarbyl group to a vanadium oxo is reported. Responsible for the C–O bond in phenol formed by the reaction of OVCl3 with HgPh2, it may provide air-regenerable metal oxos a role in aerobic alkane oxidations.

 Please wait while we load your content...
Please wait while we load your content...