The extraordinary catalytic ability of peroxiredoxins: a combined experimental and QM/MM study on the fast thiol oxidation step†
Abstract
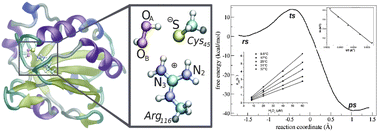
Peroxiredoxins (Prxs) catalyze the reduction of peroxides, a process of key relevance in a variety of cellular processes. The first step in the catalytic cycle of all Prxs is the oxidation of a cysteine residue to sulfenic acid, which occurs 103–107 times faster than in free cysteine. We present an experimental kinetics and hybrid QM/MM investigation to explore the reaction of Prxs with H2O2 using alkyl hydroperoxide reductase E from Mycobacterium tuberculosis as a Prx model. We report for the first time the thermodynamic activation parameters of H2O2 reduction using Prx, which show that protein significantly lowers the activation enthalpy, with an unfavourable entropic effect, compared to the uncatalyzed reaction. The QM/MM simulations show that the remarkable catalytic effects responsible for the fast H2O2 reduction in Prxs are mainly due to an active-site arrangement, which establishes a complex hydrogen bond network activating both reactive species.

 Please wait while we load your content...
Please wait while we load your content...