Palladium-catalyzed thiolation of alkanes and ethers with arylsulfonyl hydrazides†
Abstract
A new method for the preparation of alkyl aryl sulfides through direct oxidative thiolation of alkanes or ethers with arylsulfonyl hydrazides using di-tert-butyl peroxide (DTBP) as an oxidant catalyzed by Pd(OAc)2 has been reported. The C–H bonds in various alkanes or ethers were successfully converted into C–S bonds to yield the corresponding sulfides in moderate to good yields.
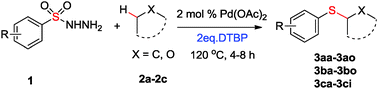

 Please wait while we load your content...
Please wait while we load your content...